View all publications via PubMed
Shah GL, Winn AN, Lin PJ, Klein A, Sprague KA, Smith HP, Buchsbaum R, Cohen JT, Miller KB, Comenzo R, Parsons SK. Cost-Effectiveness of Autologous Hematopoietic Stem Cell Transplantation for Elderly Patients with Multiple Myeloma using the Surveillance, Epidemiology, and End Results-Medicare Database. Biol Blood Marrow Transplant. 2015 Oct;21(10):1823-9.
Schubert SM1, Arendt LM2, Zhou W2, Baig S1, Walter SR1, Buchsbaum RJ3, Kuperwasser C2, Walt DR1. Ultra-sensitive protein detection via Single Molecule Arrays towards early stage cancer monitoring. Sci Rep. 2015 Jun 8;5:11034.
Buchsbaum RJ1, Oh SY2 Breast Cancer-Associated Fibroblasts: Where We Are and Where We Need to Go, Cancers (Basel). 2016 Jan 27;8(2). pii: E19. doi: 10.3390/cancers8020019.
Xu K1, Tian X2, Oh SY3,4, Movassaghi M5, Naber SP6, Kuperwasser C7,8, Buchsbaum RJ9,10. The fibroblast Tiam1-osteopontin pathway modulates breast cancer invasion and metastasis.
Breast Cancer Res. 2016 Jan 28;18(1):14
Xu K1, Buchsbaum RJ., Isolation of mammary epithelial cells from three-dimensional mixed-cell spheroid co-culture, J Vis Exp. 2012 Apr 30;(62). pii: 3760.
Liu J1, Xu K, Chase M, Ji Y, Logan JK, Buchsbaum RJ., Tiam1-regulated osteopontin in senescent fibroblasts contributes to the migration and invasion of associated epithelial cells., J Cell Sci. 2012 Jan 15;125(Pt 2):376-86
Liu J, Xu K, Chase M, Ji Y, Logan JK, Buchsbaum RJ. 2012. Tiam1-regulated osteopontin in senescent fibroblasts contributes to the migration and invasion of associated epithelial cells. J Cell Science. Epub ahead of print. Abstract
Xu K and Buchsbaum RJ. 2012. Isolation of mammary epithelial cells from three-dimensional mixed-cell spheroid co-culture. J Vis Exp, in press.
Xu K, Rajagopal S, Klebba I, Dong S, Ji Y, Liu J, Kuperwasser C, Garlick JA, Naber SP, Buchsbaum RJ. 2010. The role of fibroblast Tiam1 in tumor cell invasion and metastasis. Oncogene 29: 6533-6542. Abstract
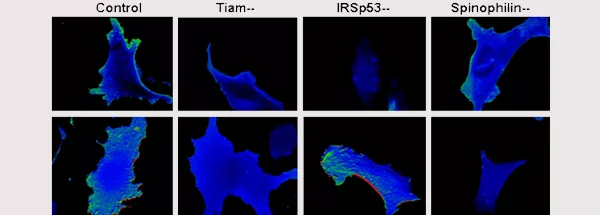
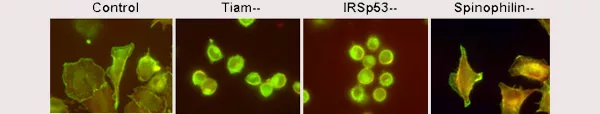
Rajagopal S, Ji Y, Xu K, Li Y, Wicks K, Liu J, Herman IM, Wong K-W, Isberg RR, and Buchsbaum RJ. 2010. Scaffold proteins IRSp53 and Spinophilin regulate localized Rac activation by Tiam1. J Biol Chem. 285: 18060-180671. Abstract
Buchsbaum RJ. 2007. Rho activation at a glance. J Cell Sci. 120: 1149-1152. Abstract
Connolly BA, Rice J, Feig LA, Buchsbaum RJ. 2005. Tiam1-IRSp53 complex formation directs specificity of rac-mediated actin cytoskeleton regulation. Mol Cell Biol 25:4602-4614. Abstract
Buchsbaum RJ, Connolly BA, Feig LA. 2003. Regulation of p70 S6 kinase by complex formation between the Rac guanine nucleotide exchange factor (Rac-GEF) Tiam1 and the scaffold spinophilin. J Biol Chem 278:18833-18841. Abstract
Buchsbaum RJ, Connolly BA, Feig LA. 2002. Interaction of Rac exchange factors Tiam1 and Ras-GRF1 with a scaffold for the p38 mitogen-activated protein kinase cascade. Mol Cell Biol 22:4073-4085. Abstract
Feig LA, Buchsbaum RJ. 2002. Cell signaling: life or death decisions of ras proteins. Curr Biol 12:R259-R261. Abstract
Buchsbaum R, Telliez JB, Goonesekera S, Feig LA. 1996. The N-terminal pleckstrin, coiled-coil, and IQ domains of the exchange factor Ras-GRF act cooperatively to facilitate activation by calcium. Mol Cell Biol 16:4888-4896. Abstract