View all publications via PubMed
Yang, E., Cisowski, J., Nguyen, N., O'Callaghan, K., Xu, Y., Agarwal, A., Kuliopulos, A., Covic, L. (2016) Oncogene 35:1529-40. Dysregulated Protease Activated Receptor 1 (PAR-1) Promotes Metastatic Phenotype in Breast Cancer through HMGA2. PMID: 26165842
Gurbel, P.G, Bliden, K.P., Turner, S.E., Tantry, U.S., Gesheff, M.G., Barr, T.P., Covic, L., Kuliopulos, A. (2016) Arterioscler Thromb Vasc Biol 36:189-197. Cell-Penetrating Pepducin Therapy Targeting PAR1 in Subjects with Coronary Artery Disease. PMID: 26681756
Shearer, A.M., Rana, R., Austin, K., Baleja, J.D., Nguyen, N., Bohm, A., Covic, L., Kuliopulos, A. (2016) J Biol Chem. 2016 Oct 28;291(44):23188-23198. Targeting Liver Fibrosis with a Cell-Penetrating PAR2 Pepducin. PMID: 2761387271.
Zhang, P., Covic, L., & Kuliopulos, A. (2015) Methods Mol Biol (2015) 1324:191-203. Pepducins and other lipidated peptides as mechanistic probes and therapeutics. PMID: 26202271.
Zhang, P., Leger, A.J., Baleja, J.D., Rana, R., Corlin, T., Nguyen, N., Koukos, G., Bohm, A., Covic., L., & Kuliopulos, A. (2015) J Biol Chem 290:15785-98. Allosteric Activation of a G Protein Coupled Receptor with Cell-Penetrating Receptor Mimetics. PMID: 25934391.
Foley, C.J., Fanjul-Fernandez, M., Bohm, A., Agarwal, A., Koukos, G., Covic, L., Lopez-Otin, C. & Kuliopulos, A. (2014) Oncogene 33:2264-72. Matrix Metalloprotease-1a Deficiency Suppresses Tumor Growth and Angiogenesis.
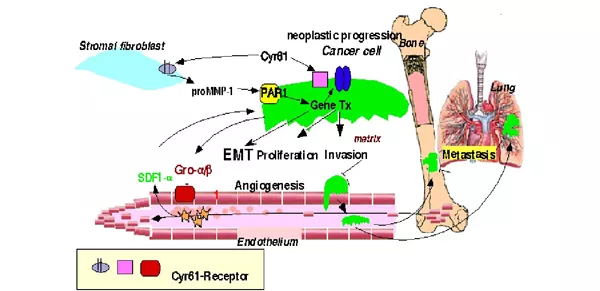
Austin, K.M., Covic, L, & Kuliopulos, A. (2013) Blood 121:431-9. Matrix Metalloproteases and PAR1 Activation. PMID: 23086754
Zhang, P., Gruber, A., Kasuda, S., Kimmelstiel, C., O’Callaghan, K., Bohm, A., Baleja, J.D., Covic, L., & Kuliopulos, A. (2012) Circulation 126:83-91. Suppression of Arterial Thrombosis with Affecting Hemostatic Parameters with a Cell-Penetrating PAR1 Pepducin. PMID: 22705889
O’Callaghan K., Kuliopulos A., Covic, L. (2012) J Biol Chem 287:12787-96. Turning Receptors On and Off with Intracellular Pepducins: New Insights into G-protein Coupled Receptor Drug Development. PMCID: PMC3339939
O’Callaghan, K., Lee, L., Nguyen, N., Hsieh, M.-Y., Kaneider, N.C., Klein, A.K., Sprague, K., Van Etten, R.A., Kuliopulos, A. Covic, L. (2012) Blood 119:1717-25. Targeting CXCR4 with cell-penetrating pepducins in lymphoma and lymphocytic leukemia. PMID: 22186993
Cisowski J, O’Callaghan K, Kuliopulos A, Yang J, Nguyen N, Deng Q, Yang E, Fogel M, Tressel S, Foley C, Agarwal A, Hunt III SW, McMurry T, Brinckerhoff L, Covic L. (2011) Amer J Pathol, 179:513-23. Targeting protease-activated receptor-1 with cell-penetrating pepducins in lung cancer. PMID: 21703428
Kimmelstiel C, Zhang P, Kapur NK, Weintraub A, Krishnamurthy B, Castaneda V, Covic L, Kuliopulos A. (2011) Circ Cardiovasc Interv. 4:171-9. Bivalirudin Is a dual inhibitor of thrombin and collagen-dependent platelet activation in patients undergoing percutaneous coronary intervention. PMID: 21364148
Tressel SL, Koukos G, Tchernychev B, Jacques SL, Covic L, Kuliopulos A. (2010) Methods Mol Biol. 683: 259-275. Pharmacology, biodistribution, and efficacy of GPCR-based pepducins in disease models. PMID: 21053136
Agarwal A, Tressel SL, Kaimal R, Balla M, Lam FH, Covic L, Kuliopulos A. (2010) Cancer Res. 70: 5880-5890. Identification of a metalloprotease-chemokine signaling system in the ovarian cancer microenvironment: Implications for antiangiogenic therapy. PMID: 20570895
Trivedi V, Boire A, Tchernychev B, Kaneider NC, Leger AJ, O'Callaghan K, Covic L, Kuliopulos A. (2009) Cell 137: 332-343. Platelet matrix metalloprotease-1 mediates thrombogenesis by activating PAR1 at a cryptic ligand site. PMID: 19379698
Nguyen N, Kuliopulos A, Graham RA, Covic L. (2006) Cancer Res. 66: 2658-2665. Tumor-derived Cyr61(CCN1) promotes stromal matrix metalloproteinase-1 production and protease-activated receptor 1-dependent migration of breast cancer cells. PMID: 16510585