The PARs are cleaved by thrombin and other proteases at a specific peptide bond to expose a new N-terminus that binds to the body of the receptor in an unusual intramolecular mode. These receptors are widely expressed throughout human tissues including blood cells, endothelium, liver, kidney, lung, skin, and invasive cancer cells. A major interest of our research group is to study the molecular mechanism of protease activation of the PARs and the subsequent signaling in vascular cells, fibrotic processes and in cancer.
Overview
Athan Kuliopulos, MD, PhD
Principal Investigator
Susan Turner, BS
Clinical Trials Manager
Rajashree Rana, PhD
Post-doctoral Fellow
Elizabeth Fletcher, PhD
Post-doctoral Fellow
Emily Michael, BS
Student
Andrew Shearer, BS
Student
View all publications via PubMed
Wieser, V., Adolph, T.E., Enrich, B., Kuliopulos, A., Kaser, A., Tilg, H., Kaneider, N.C. (2017) Gut May;66(5):930-938. Reversal of murine alcoholic steatohepatitis by pepducin-based functional blockade of interleukin-8 receptors.
Shearer, A.M., Rana, R., Austin, K., Baleja, J.D., Nguyen, N., Bohm, A., Covic, L., Kuliopulos, A. (2016) J Biol Chem. 2016 Oct 28;291(44):23188-23198. Targeting Liver Fibrosis with a Cell-Penetrating PAR2 Pepducin. PMID: 2761387271.
Harshman, S.G., Fu, X., Karl, J.P., Lamon-Fava, S., Kuliopulos, A., Greenberg, A., Smith, D., Shen, X., Booth, S.L. J Nutr. (2016) 146(8):1521-7. Tissue concentrations of vitamin K and expression of key enzymes of vitamin K metabolism are influenced by sex and diet, but not housing in C57Bl6 mice. PMID: 27385762.
Gurbel, P.G, Bliden, K.P., Turner, S.E., Tantry, U.S., Gesheff, M.G., Barr, T.P., Covic, L., Kuliopulos, A. (2016) Arterioscler Thromb Vasc Biol 36:189-197. Cell-Penetrating Pepducin Therapy Targeting PAR1 in Subjects with Coronary Artery Disease. PMID: 26681756.
Zhang P, Covic L, Kuliopulos A. 2015. Pepducins and other lipidated peptides as mechanistic probes and therapeutics. Methods Mol Biol. 1324: 191-203.
Yang E, Cisowski J, Nguyen N, O'Callaghan K, Xu J, Agarwal A, Kuliopulos A, Covic L. 2015. Dysregulated protease activated receptor 1 (PAR1) promotes metastatic phenotype in breast cancer through HMGA2. Oncogene 35: 1529-1540.
Zhang P, Leger AJ, Baleja JD, Rana R, Corlin T, Nguyen N, Koukos G, Bohm A, Covic L, Kuliopulos A. 2015. Allosteric activation of a G protein coupled receptor with cell-penetrating receptor mimetics. J Biol Chem. 290: 15785-15798.
Gurbel PA, Kuliopulos A, Tantry US. 2015. G-protein-coupled receptors signaling pathways in new antiplatelet drug development. Arterioscler Thromb Vasc Biol.35: 500-512 .
Mouse matrix metalloprotease-1a (Mmp1a) gives new insight into MMP function. Foley CJ, Kuliopulos A. J Cell Physiol. 2014 Dec;229(12):1875-80 [PubMed - indexed for MEDLINE] Free PMC Article
Noncanonical matrix metalloprotease-1-protease-activated receptor-1 signaling triggers vascular smooth muscle cell dedifferentiation and arterial stenosis. Austin KM, Nguyen N, Javid G, Covic L, Kuliopulos A. J Biol Chem. 2013 Aug 9;288(32):23105-15. Free PMC Article
Matrix metalloprotease 1a deficiency suppresses tumor growth and angiogenesis. Foley CJ, Fanjul-Fernández M, Bohm A, Nguyen N, Agarwal A, Austin K, Koukos G, Covic L, López-Otín C, Kuliopulos A. Oncogene. 2014 Apr 24;33(17):2264-72. Free PMC Article
Selective blockade of matrix metalloprotease-14 with a monoclonal antibody abrogates invasion, angiogenesis, and tumor growth in ovarian cancer. Kaimal R, Aljumaily R, Tressel SL, Pradhan RV, Covic L, Kuliopulos A, Zarwan C, Kim YB, Sharifi S, Agarwal A. Cancer Res. 2013 Apr 15;73(8):2457-67. Free PMC Article
Pharmacological inhibition of PAR2 with the pepducin P2pal-18S protects mice against acute experimental biliary pancreatitis. Michael ES, Kuliopulos A, Covic L, Steer ML, Perides G. Am J Physiol Gastrointest Liver Physiol. 2013 Mar 1;304(5):G516-26. Free PMC Article
Matrix metalloproteases and PAR1 activation. Austin KM, Covic L, Kuliopulos A. Blood. 2013 Jan 17;121(3):431-9. doi: 10.1182/blood-2012-09-355958. Free PMC Article
Suppression of arterial thrombosis without affecting hemostatic parameters with a cell-penetrating PAR1 pepducin. Zhang P, Gruber A, Kasuda S, Kimmelstiel C, O'Callaghan K, Cox DH, Bohm A, Baleja JD, Covic L, Kuliopulos A. Circulation. 2012 Jul 3;126(1):83-91. Free PMC Article
Matrix metalloprotease-1a promotes tumorigenesis and metastasis. Foley CJ, Luo C, O'Callaghan K, Hinds PW, Covic L, Kuliopulos A. J Biol Chem. 2012 Jul 13;287(29):24330-8. Free PMC Article
Turning receptors on and off with intracellular pepducins: new insights into G-protein-coupled receptor drug development. O'Callaghan K, Kuliopulos A, Covic L. J Biol Chem. 2012 Apr 13;287(16):12787-96. Free PMC Article
O'Callaghan K, Lee L, Nguyen N, Hsieh MY, Kaneider NC, Klein AK, Sprague K, Van Etten RA, Kuliopulos A, Covic L. 2012. Targeting CXCR4 with cell-penetrating pepducins in lymphoma and lymphocytic leukemia. Blood. 119(7): 1717-25. Abstract
Sevigny LM, Austin KM, Zhang P, Kasuda S, Koukos G, Sharifi S, Covic L, Kuliopulos A. 2011. Protease-activated receptor-2 modulates protease-activated receptor-1-driven neointimal hyperplasia. Arterioscler Thromb Vasc Biol. 12: e100-6. Abstract
Sevigny LM, Zhang P, Bohm A, Lazarides K, Perides G, Covic L, Kuliopulos A. 2011. Interdicting protease-activated receptor-2-driven inflammation with cell-penetrating pepducins. Proc Natl Acad Sci U S A. 108(20): 8491-6. Abstract
Tressel SL, Kaneider NC, Kasuda S, Foley C, Koukos G, Austin K, Agarwal A, Covic L, Opal SM, Kuliopulos A. 2011. A matrix metalloprotease-PAR1 system regulates vascular integrity, systemic inflammation and death in sepsis. EMBO Mol Med. 3(7): 370-84. Abstract
Kimmelstiel C, Zhang P, Kapur NK, Weintraub A, Krishnamurthy B, Castaneda V, Covic L, Kuliopulos A. 2011. Bivalirudin Is a dual inhibitor of thrombin and collagen-dependent platelet activation in patients undergoing percutaneous coronary intervention. Circ Cardiovasc Interv. 4: 171-179. Abstract
Tressel SL, Koukos G, Tchernychev B, Jacques SL, Covic L, Kuliopulos A. 2011. Pharmacology, biodistribution, and efficacy of GPCR-based pepducins in disease models. Methods Mol Biol. 683: 259-275. Abstract
Agarwal A, Tressel SL, Kaimal R, Balla M, Lam FH, Covic L, Kuliopulos A. 2010. Identification of a metalloprotease-chemokine signaling system in the ovarian cancer microenvironment: Implications for antiangiogenic therapy. Cancer Res. 70: 5880-5890. Abstract
Trivedi V, Boire A, Tchernychev B, Kaneider NC, Leger AJ, O'Callaghan K, Covic L, Kuliopulos A. 2009. Platelet matrix metalloprotease-1 mediates thrombogenesis by activating PAR1 at a cryptic ligand site. Cell 137: 332-343. Abstract
Kaneider NC, Leger AJ, Agarwal A, Nguyen N, Perides G, Derian C, Covic L, Kuliopulos A. 2007. 'Role reversal' for the receptor PAR1 in sepsis-induced vascular damage. Nat Immunol. 8: 1303-1312. Abstract
Kaneider NC, Leger AJ, Kuliopulos A. 2006. Therapeutic targeting of molecules involved in leukocyte-endothelial cell interactions. FEBS J. 273: 4416-4424. Abstract
Leger AJ, Jacques SL, Badar J, Kaneider NC, Derian CK, Andrade-Gordon P, Covic L, Kuliopulos A. 2006. Blocking the protease-activated receptor 1-4 heterodimer in platelet-mediated thrombosis. Circulation 113: 1244-1254. Abstract
Kaneider NC, Agarwal A, Leger AJ, Kuliopulos A. 2005. Reversing systemic inflammatory response syndrome with chemokine receptor pepducins. Nat Med. 11: 661-665. Abstract
Boire A, Covic L, Agarwal A, Jacques S, Sherifi S, Kuliopulos A. 2005. PAR1 is a matrix metalloprotease-1 receptor that promotes invasion and tumorigenesis of breast cancer cells. Cell 120: 303-313. Abstract
Research focus areas
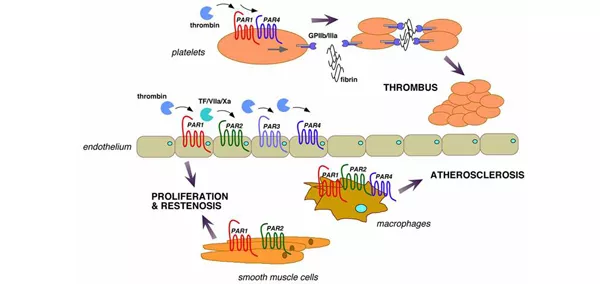
Thrombosis associated with the pathophysiological activation of platelets and vascular cells has brought thrombin and its receptors to the forefront of cardiovascular medicine. Protease signaling through the protease-activated receptors (PARs) has been shown to influence a wide range of physiological responses including platelet activation, intimal hyperplasia, inflammation, and maintenance of vascular tone and barrier function. A major goal of our Center of Hemostasis and Thrombosis Research is to determine the efficacy and safety of experimental anti-thrombotic agents in patients. We conduct Phase 1, Phase 2 and Phase 3 and translational studies which serve as the basis for improving the outcome, safety, and dosage of various drugs to most effectively treat patients with severe heart disease and myocardial infarction.
Emerging evidence also suggests that inappropriate matrix metalloprotease (MMP) activity may underlie the pathogenesis of atherosclerosis and vascular inflammation. Despite data that MMPs contribute to atherosclerotic lesion remodeling and poor outcomes, essentially nothing is known regarding the role of MMPs as active signaling molecules in controlling the behavior of vascular cells in the context of atherosclerotic disease. We recently made the unanticipated discovery that MMP-1 cleaves and activates protease-activated receptor-1 (PAR1) signaling in blood vessels. This is of major import as it was the first report of a direct signaling function of the principal collagenase in blood vessels. Notably, we found that MMP-1 activates PAR1 by cleaving the receptor at a distinct site from the canonical thrombin cleavage site which generates a longer tethered ligand that is biased towards a different spectrum of G protein signaling pathways. The studies in this project focus on the completely unexplored role of MMP1-PAR1 in the development of atherosclerotic plaques and understanding the pathophysiologic relevance of MMP1-PAR1 signaling on endothelium and macrophages. The mechanism linking these events to atherosclerotic plaque development will provide a framework to advance future therapies that could halt or reverse the progression of atherosclerosis.
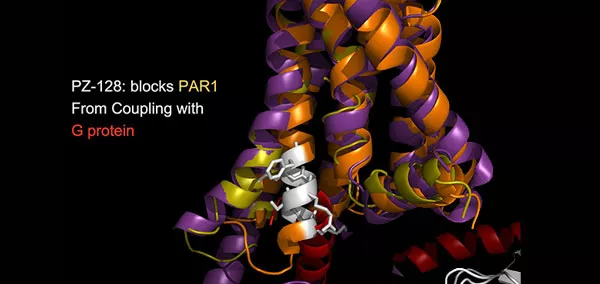
We have discovered and developed a new class of cell-penetrating, membrane-tethered peptides—named pepducins—that are based on the intracellular loops of receptors. Pepducins rapidly flip across the plasma membrane and cause full activation or inhibition of G protein-dependent signaling. Most remarkably, pepducins are selective for their cognate receptor and require receptor for activity both in cell culture and in animals. Drugs based on pepducins may prove to be the first therapeutically useful agents targeted to the receptor-G protein interface. Pepducins can also be used to define the roles of orphan receptors which lack known agonists or antagonists and can help define the complex pharmacologies of receptors whose functional interactions and signaling change over time. We are currently testing PZ-128 as a fast-acting PAR1 pepducin inhibitor in a multi-center phase 2 clinical trial which may confer significant clinical benefits to patients at high risk for life-threatening arterial thrombosis and myocardial infarction.