According to the American Cancer Society, about 1 in 7 men will be diagnosed with prostate cancer during their lifetimes. Although it is a serious disease, most men diagnosed with prostate cancer do not die from it – more than 2.9 million men in the U.S. are currently living with prostate cancer.
Overview
The largest source of illness and death associated with prostate cancer happens when the cancer spreads (metastasizes) to the bones. The bones are almost always where prostate cancer spreads first. When this occurs, it can be very painful and cause life-threatening problems, including spinal cord damage or failure of blood production.
After prostate cancer has spread to the bones, the goal of treatment becomes focused not only on slowing the spread of the cancer but also controlling or relieving pain. Treatments often used include chemotherapy, vaccines, hormone therapy and drugs like bisphosphonates, denosumab and corticosteroids.
At Tufts Medical Center’s Cancer Center in Boston, MA, we have top oncologists who specialize not only in treating prostate cancers and bone metastases, but also in furthering our understanding of the biological mechanisms behind this behavior so that we can develop innovative solutions to prevent or slow the process.
Paul Mathew, MD, an oncologist and researcher in the Molecular Oncology Research Institute at Tufts MC, trained at the Cook County Hospital and the Mayo Clinic, has cared for patients at world-renowned centers like MD Anderson, and is a regular Boston Magazine “Top Doctor.” He also leads a research laboratory at Tufts MC that is focused on experimental therapeutics in prostate cancer and modeling bone metastases.
Dr. Mathew’s lab at has recently been able to pinpoint a mechanism that might explain how prostate cancer spreads to the bones. They identified a key process that occurs when fibronectin (an adhesive molecule that connects cells to other components like collagen) fragments in the bone marrow signal to a receptor in prostate cancer cells called the integrin alpha 5 beta 1 heterodimer.
This mechanism follows a hypothesis called the “seed-and-soil mechanism,” which was originally developed in 1889 by Stephen Paget, an English surgeon and pathologist. Paget believed that the spread of cancer did not occur randomly, but instead only metastasized when the seed (the cancer cell) and the soil (in this case, the fibronectin fragments in the bone marrow) are compatible.
Dr. Mathew’s discovery of the presence of this pathway in prostate cancer’s spread to the bone has huge implications for improving options to slow or even prevent this mechanism entirely. Specifically, this explanation signals the need to advance our use of molecular therapeutics so that we can prevent the “soil” (fibronectin fragments) from signaling to the “seed” (prostate cancer cells). Dr. Mathew’s lab is working on such research now. For example, blocking the integrin alpha 5 beta 1 heterodimer with a monoclonal antibody could significantly slow or prevent the progression of bone metastases.
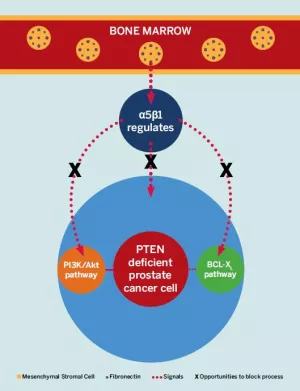
Recently, Dr. Mathew’s team has corroborated their “seed-and-soil mechanism” theory for prostate cancer spread by looking further downstream of alpha 5 integrin signaling which connects to a mutation in a gene within the pro-survival pathway of prostate cancer cells. “This gene, PTEN, is one of the most commonly mutated tumor suppressors in human cancer. The fact that PTEN mutates in prostate cancer cells is particularly important in the progression of prostate cancer to higher grade and stage, including metastases,” said Dr. Mathew.
The discovery of pro-survival pathways in prostate cancer cells is a major breakthrough in prostate cancer research. Dr. Mathew’s lab has uncovered that a simultaneous blockage of two of these pathways (PI3Kinase/Akt and BCL-XL) results in what is referred to as programmed cell death or apoptosis. This means that by using “synthetic lethality” (when a combination of mutations in two or more genes leads to cell death), we could develop treatments to kill prostate cancer cells when PTEN has been inactivated.
“This represents a novel treatment strategy for prostate cancers in which PTEN is inactivated, which represents at least 50% of advanced metastatic prostate cancer,” Dr. Mathew explained.
By improving our understanding of how prostate cancer cells signal to the bone marrow and move through pro-survival pathways, Dr. Mathew’s lab has taken a huge step forward in the field of prostate cancer research.
The next step is testing the theory that using precision medicine and synthetic lethality to block pro-survival pathways will slow the progression and deadliness of prostate cancer. The results of such a study could have huge implications for millions of men in the United States and worldwide.
To support these studies and Dr. Mathew’s research, visit our Giving + Support form, choose “Other” and designate your gift to "Dr. Paul Mathew’s Prostate Research Fund."