Current projects include:
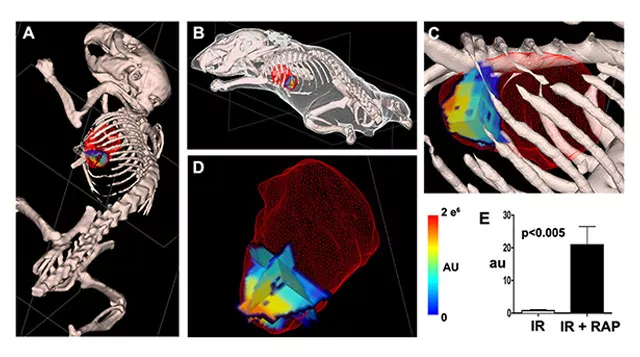
Molecular imaging of cell death, namely autophagy, necrosis and apoptosis, relies on intravenously injectable contrast agents to target specific biological states and provide sensitive readout. The ability to image cell death processes noninvasively in vivo allows the kinetics to be studied during disease progression and regression. Autophagy is a conserved catabolic process where cells recycle part of the cytoplasm during stress. Interestingly, autophagy can exert either detrimental or protective pathophysiological effects on the heart during disease. We demonstrated for the first time probe-based imaging of autophagy in a beating mouse heart. In mouse hearts with ischemia-reperfusion injury (IR), autophagy induction by Rapamycin (RAP) was visualized and quantified (Figure 1).
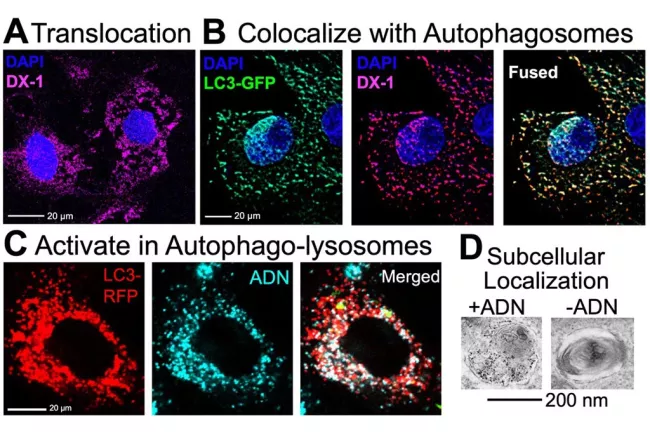
The Autophagy imaging probe mechanism is further elucidated in cell culture (Figure 2). Probe activation following autophagy induction is three-fold: i) the rapid translocation of the autophagy probe into the cytoplasm, ii) the high degree of co-trafficking between the autophagy probe and early autophagosomes and iii) the activatable nature of the autophagy probe upon entering autophagolysosomes. We hypothesize that bioconjugation chemistry strategies can be applied to engineer second-generation autophagy sensing probes with enhanced sensitivity and specificity to monitor autophagy levels non-invasively in vivo.
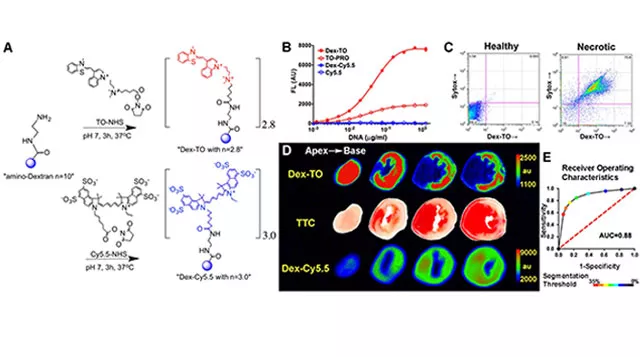
Theranostics are imaging agents with dual therapeutic and diagnostic capabilities. Developing theranostics would allow us to see disease processes non-invasively in the body, simultaneously applying therapeutics at the site of injury, and yield real-time readout of response to treatment. An example of a theranostic nanoprobe is a biocompatible polymer construct engineered with nucleic acid chelating fluorochromes. The probe targets nucleic acids released during cell rupture to facilitate imaging of cardiomyocyte necrosis (Figure 3).
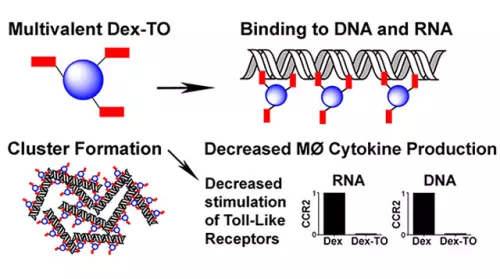
Extracellular nucleic acids are robust proinflammatory molecules that elicit immune cell infiltration. Theranostic nucleic acid binding nanoprobe developed has nanomolar affinity for mammalian and bacterial nucleic acids and attenuates the production of inflammatory cytokines from activated macrophages exposed to DNA or RNA (Figure 4). In mice treated with the nanoprobe, inflammation was reduced at 24 hours after myocardial ischemia-reperfusion injury, leading to improved cardiac healing by day 7. Food and Drug Administration (FDA)-approved biocompatible polymer platform further allows pharmacokinetics to be optimized to enhance diagnostic and therapeutic efficacies and can be translatable from molecular targeting to improve treatment outcomes.
Lab members
- Asma Boukhalfa, Postdoctoral Fellow
Previous members
- Andrew Kung, Undergraduate Researcher
- Ada Yu, Undergraduate Researcher
- Fernanda Garcia, Undergraduate Researcher