Current projects include:
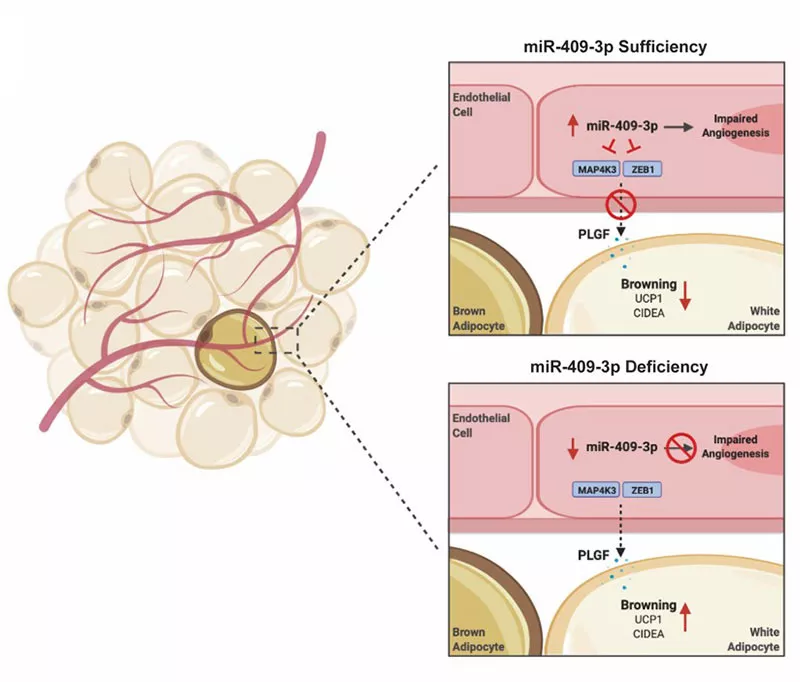
Endothelial cells (ECs) within the microvasculature of brown adipose tissue (BAT) are important in regulating the plasticity of adipocytes in response to increased metabolic demand by modulating the angiogenic response. However, the mechanism of EC-adipocyte crosstalk during this process is not completely understood. Using a number of profiling approaches, we have identified miR-409-3p and its targets ZEB1 and MAP4K3 as potent regulators of angiogenesis in obesity. MiR-409-3p neutralization improved BAT angiogenesis, glucose and insulin tolerance, and energy expenditure in mice with diet-induced obesity through EC-adipocyte cross-talk via endothelial release of the pro-angiogenic growth factor, placental growth factor (PLGF). Our findings establish miR-409-3p as a critical regulator of EC-BAT crosstalk by modulating a ZEB1-MAP4K3-PLGF signaling axis, providing new insights for therapeutic intervention in obesity (Icli and colleagues, CMLS, PMID 34698882)
Angiogenesis is critical for tissue repair following myocardial ischemia, diabetic wound healing, processes by which are exacerbated under insulin resistance or diabetes. MicroRNAs are regulators of angiogenesis. Using human plasma samples with acute coronary syndrome (ACS), genetic deletion of microRNA and human endothelial cells, we show that miR-409-3p improves angiogenesis and heart function in mice post-MI and targets the DNAJB9/p38 mitogen-activated protein kinase (MAPK) signaling pathway. Our findings unveil possible targets for future therapeutic modulation to improve angiogenesis and remodeling of the heart post-MI. (Icli and colleagues, MTNA)

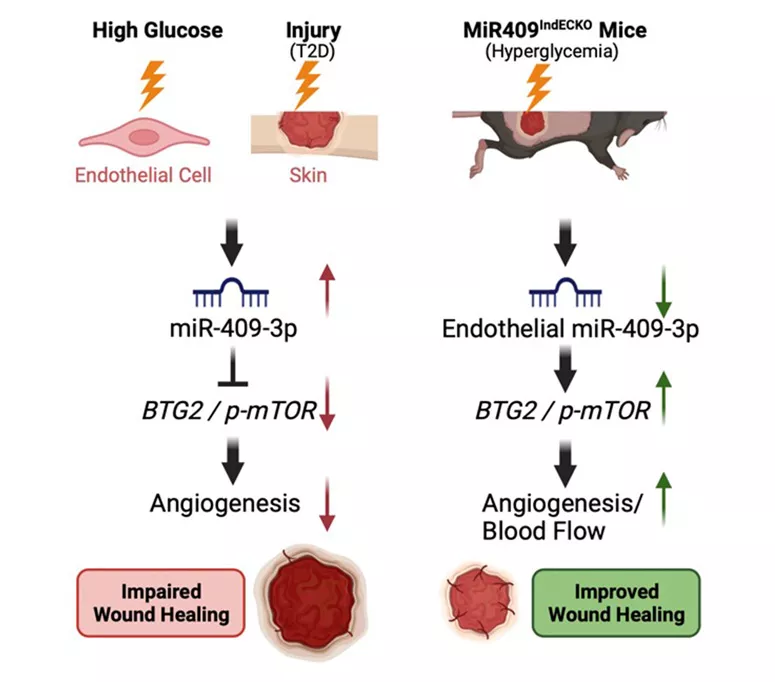
Furthermore, endothelial-specific and tamoxifen-inducible miR-409-3p knock-out mice (MiR-409IndECKO) with hyperglycemia that underwent dorsal skin wounding showed significant improvement of wound closure, increased blood flow, granulation tissue thickness (GTT), and CD31 while significantly decreasing mTOR phosphorylation. (Icli and colleagues, FASEB J-in press)
Lab members
- Furkan Bestepe, MD, Postdoctoral Research Fellow
- George Ghanem, BS, Research Assistant
- Parul Sahu, PhD, Postdoctoral Research Fellow
- Sezan Vehbi, MD, Visiting Research Scholar