The Kapur Lab was the first to establish that reduced endoglin activity improves survival and limits maladaptive cardiac remodeling in heart failure. The laboratory identified that targeting endoglin using an antibody-mediated approach limits and reverses established cardiac fibrosis in preclinical models of heart failure and acute myocardial infarction. The Kapur Lab was the first to identify that acute mechanical unloading of the left ventricle activates a cardioprotective signaling program in the setting of acute myocardial infarction and the first to identify novel molecular mechanisms regulating cardiac biology and mitochondrial function with extracorporeal membrane oxygenation (ECMO). Dr. Kapur is an inventor on multiple issued patents several of which have formed the basis for new companies.
Current projects include:
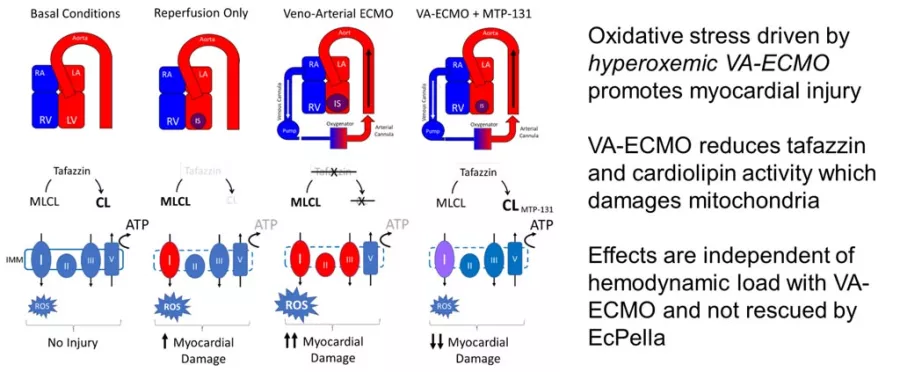
The Kapus Laboratory was the first to identify that reducing left ventricular (LV) wall stress, known as LV unloading, with percutaneously-delivered mechanical heart pumps and intentionally delaying coronary reperfusion significantly reduces infarct size in models of acute myocardial infarction (AMI). Using unbiased genomic and metabolomic screening approaches, we identified that LV unloading before reperfusion promotes a global shift in myocardial biology that limits reperfusion injury by promoting cardioprotective signaling and preserving mitochondrial integrity. Using conductance catheter methods and coronary wave intensity analysis we were the first to illustrate a link between LV unloading and microvascular perfusion with trans-valvular axial flow pumps and veno-arterial extracorporeal membrane oxygenation (VA-ECMO). This work led to the STEMI-Door to Unload Pilot Trial, which confirmed the safety, feasibility, and potential efficacy of percutaneously unloading the LV and delaying coronary reperfusion in patients with STEMI. This landmark pilot study led to the STEMI-DTU Pivotal trial, for which I serve as the global principal investigator. Our recent work focuses on novel physiologic and biologic approaches to limit cardiac and vascular injury in the setting of VA-ECMO. We are the first to identify that VA-ECMO disrupts mitochondrial integrity by depleting myocardial tafazzin and cardiolipin levels.
The laboratory has been focused on advancing our understanding of cardiogenic shock. My laboratory was the first to describe the pulmonary artery pulsatility index (PAPI), a novel formula to assess right heart function, that is now included in multiple consensus statements. In addition to reporting findings from clinical conductance catheter studies in cardiogenic shock, I founded and directed the Cardiogenic Shock Working Group, which is an academic research consortium of 40 hospitals in the United States, Europe, Japan, Mexico and Australia (Figure). I launched the CSWG registry in 2017 which now includes >10,000 patients with an accrual rate of >2000 new patients annually. The CSWG was the first to identify phenotypes of cardiogenic shock (Figure) and is now implementing advanced data analytics and machine learning methods to develop a clinical decision support system to improve patient outcomes.

The laboratory has been involved in the testing and development of multiple novel circulatory support devices including pneumatic, rotary-flow, or membrane-based intracorporeal and extracorporeal pumps. Several of these technologies have successfully bridged into clinical trials including the HeartMate PHP, Aortix pump, Protek Duo, Impella ECP, and Impella RP. I also co-founded and launched an award-winning company known as Precardia (Figure), which is developing a novel device to mechanically regulate cardiac preload thereby reducing both cardiac workload and renal afterload. Precardia was acquired in 2021.

Lab members
- Shreyas Bhave, PhD, Postdoctoral Research Fellow
- Christian Canales, CVT, Research Associate
- Kay Everett, MD, PhD, Assistant Professor of Medicine
- Elena Mahmoudi, DVM, Research Associate
- Xiaoying Qiao, MD, PhD Research Associate
- Lara Reyelt, BS, CVT, SRS, Special and Scientific Staff
- Lija Swain, PhD, Instructor in Medicine